schoolМатематика
verifiedРешено AI
Решение Задания 8 по Химии
help_outlineУсловие задачи
lightbulbКраткий ответ
Представлены верные ответы на задание 8 из школьного учебника химии. Решение оформлено для удобного использования при выполнении домашней работы.
Подробное решение
Ниже представлены решения заданий из ваших учебных материалов, оформленные для удобного переписывания в тетрадь.
Задание 8. Выберите правильное окончание предложения.
1. Chancourtois arranged elements
Ответ: c) on a helical curve
2. The first step in classification of elements was taken by
Ответ: b) Dobereiner
3. The final and most important step in the development of the periodic table was made by
Ответ: b) Mendeleyev
4. The Dutch physicist van den Brock suggested that
Ответ: b) the nuclear charge of an element might be equal to the ordinal number of the element in the periodic system
5. The periodic law was accepted immediately after its proposal by Mendeleyev because
Ответ: c) of his success in making predictions with its use which were afterward verified by experiment.
Задание 13. Найдите в тексте слова со следующими значениями.
1. "laugh at" — ridicule
2. "to examine something and make corrections in it" — reconstruct
3. "mistake" — error
4. "changed in order or position" — rearranged
5. "anxiety, worry" — concern (или "much concern" из контекста)
6. "importance" — value / greatness
7. "nuclear charge" — atomic number
Задание 14. Заполните пропуски словами из текста.
1. Newlands called his relation the law of octaves, by analogy with the seven intervals of the musical scale.
2. In 1871 Mendeleyev and Meyer each proposed a table with eight columns, obtained by dividing each of the long periods into periods of seven elements.
3. Further experimental work proved that Mendeleyev's predictions were correct.
4. A long time was needed for the recognition of the fact that the elements can be classified in the way now described by a periodic table.
5. This change of order from that of atomic weight caused much concern.
Задание 18. Соотнесите русские и английские эквиваленты.
1. to be born — е. родиться
2. to graduate from — а. окончить
3. enormous amount — д. огромное количество
4. in the order of — б. в порядке
5. repetition of properties — в. повторяемость свойств
6. to overcome difficulties — г. преодолеть трудности
Задание 19. Расположите параграфы в логическом порядке.
Правильная последовательность: 11, 3, 6, 7, 5, 4, 8, 9, 10, 2, 1.
Задание 20. Выберите правильный ответ о величии открытия Менделеева.
Ответ: 1. The greatness of Mendeleyev's achievement lies in the fact that his Periodic Table pointed the way to further progress in chemistry.
Задание 21. Ответьте на вопросы.
1. D.I. Mendeleyev was born in 1834 in Tobolsk.
2. He studied chemistry at the Pedagogical Institute in St. Petersburg and later in Germany.
3. One of his very important books is "Principles of Chemistry" (or "Fundamentals of Chemistry").
4. His greatest discovery is the Periodic Law of chemical elements.
5. He was interested in coal, petroleum, iron, and steel industries.
6. The Periodic Law has preserved its value because it is a fundamental law of nature that allows scientists to predict the properties of new elements and unify chemical knowledge.
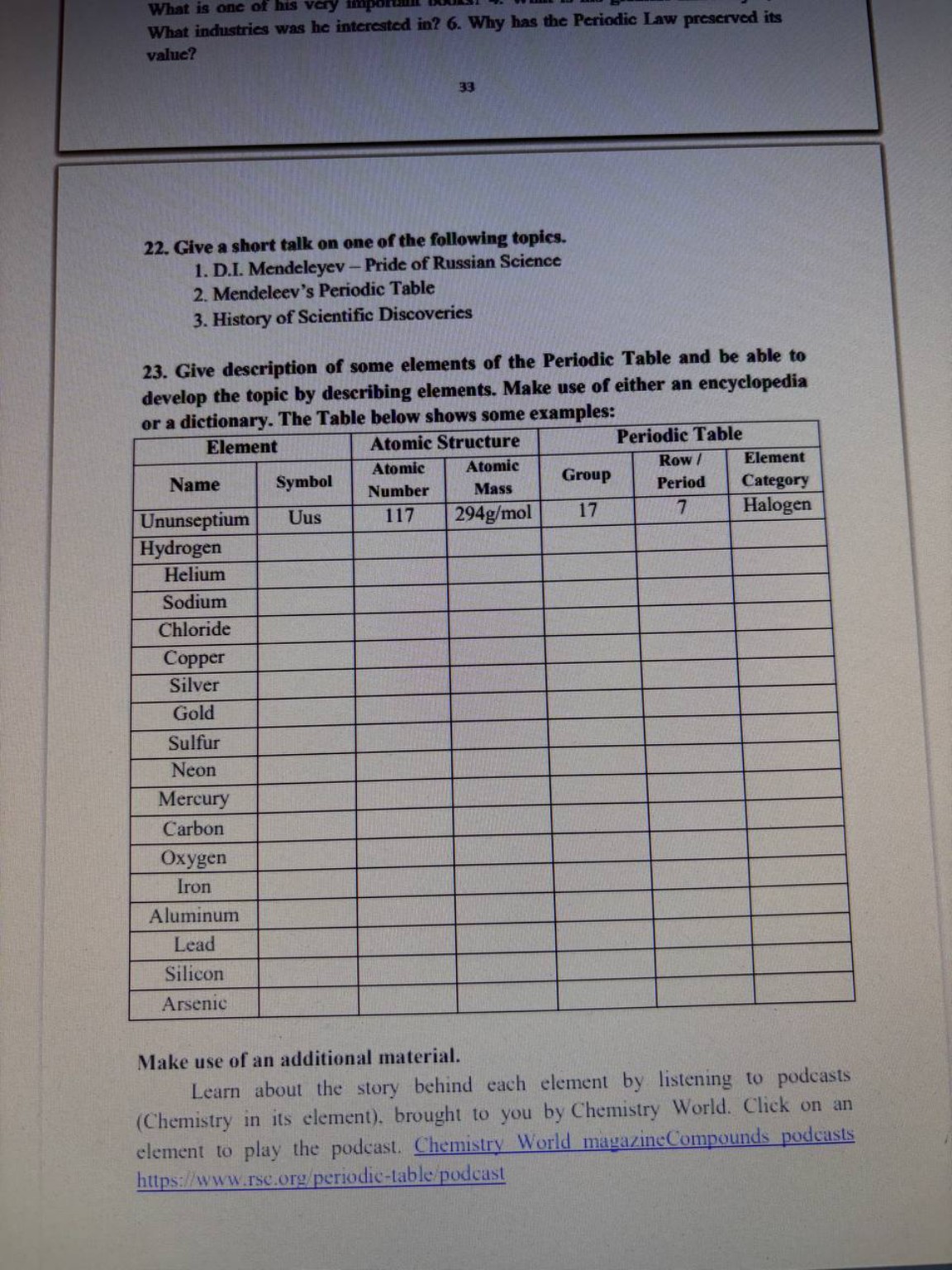
Задание 23. Заполнение таблицы (примеры элементов).
1. Hydrogen: Symbol \(H\), Atomic Number \(1\), Atomic Mass \(1.008\), Group \(1\), Period \(1\), Category: Non-metal.
2. Helium: Symbol \(He\), Atomic Number \(2\), Atomic Mass \(4.002\), Group \(18\), Period \(1\), Category: Noble gas.
3. Sodium: Symbol \(Na\), Atomic Number \(11\), Atomic Mass \(22.99\), Group \(1\), Period \(3\), Category: Alkali metal.
4. Iron: Symbol \(Fe\), Atomic Number \(26\), Atomic Mass \(55.84\), Group \(8\), Period \(4\), Category: Transition metal.
5. Oxygen: Symbol \(O\), Atomic Number \(8\), Atomic Mass \(15.99\), Group \(16\), Period \(2\), Category: Non-metal.